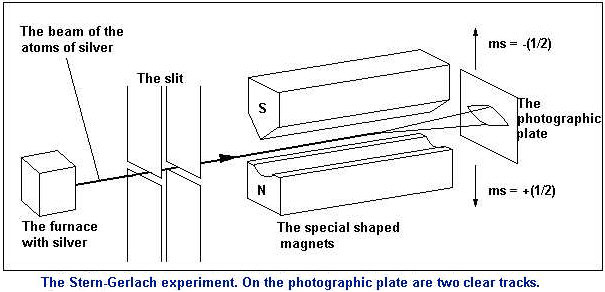
When you for the first time encounter magnets (when you were a child or so) it is clear they are magical things. When you as a reader are still young and later in life you get kids too, always make sure there are a few magnets in their collection of toys. (And also ensure there is enough simple plain version of Lego, later in file this is good for their geometrical insights.)
Let me first summarize how permanent magnets are made:
- Pieces of metal are heated until they get above the so called Curie temperature.
- The pieces of metal are fixated in place and a strong magnetic field is applied, very slowly the pieces of metal are cooled down.
- Hammering the metal while it cools down seems to help a lot.
- When cooled down the magnets are ready to use but they often get a sanding and a paint job to make them look nice and add a layer that prevents rust.
What is the Curie temperature?
Answer: That is the temperature when you heat a permanent magnet above that temperature it will loosed all of it’s (permanent) magnetism.
The existence of such a Curie temperature is also in favor of my version of how permanent magnets work but let me build it up slow and steadily and not bring you into confusion.
Now the professional professors know that it are the unpaired electrons that are the root cause of permanent magnetism. Here is a picture of how those professionals think it is, all magnetic metals have so called domains inside their crystalline structure and this is more or less schematic how it is supposed to work:
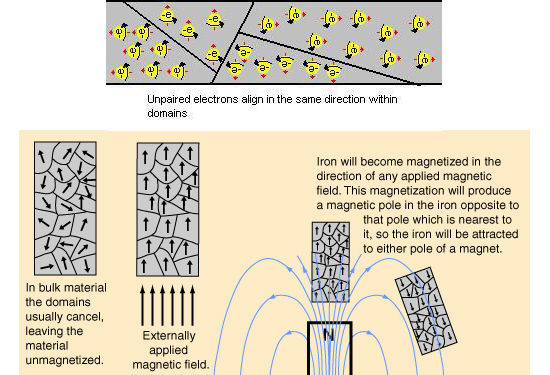 In the upper part of the picture you see that even after a full century a lot of people still think the electrons are actually spinning, but why should electrons spin frantically with a precise speed anyway? Also in the above picture you see the habit of using vectors to represent magnetic dipole moment, that is ok only not on the level of individual electrons.
In the upper part of the picture you see that even after a full century a lot of people still think the electrons are actually spinning, but why should electrons spin frantically with a precise speed anyway? Also in the above picture you see the habit of using vectors to represent magnetic dipole moment, that is ok only not on the level of individual electrons.
It is good that professional physics people pointed out the unpaired electrons, but they still think that those electrons themselves are magnetic dipoles. Here they have a giant problem they never talk about: if an unpaired electron is a magnetic dipole, it is obvious that an electron pair is also a magnetic dipole. But why do electron pairs never contribute to macroscopic magnetism?
__________
Ok, now we use my version of reality and in my version of reality electrons always carry a negative electric charge and each electron carries also one of the two magnetic charges there are: north charge or south charge.
This explains electron pair formation in the first place but this post is not about electron pair formation instead we try to understand all those metals that can have permanent magnetism.
What all those metals that can be permanently magnetized have in common is very easy to understand: They have lots of unpaired electrons below the outer electron shells.
The best picture I could find was this (the electron pair in the most outward shell is not realistic of course, thar violates the so called ‘Aufbou prinzip’ but it was the best picture I could find):
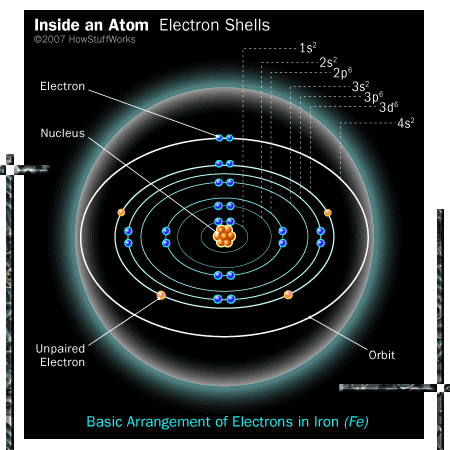 You see the unpaired electrons inside the iron atom.
You see the unpaired electrons inside the iron atom.
Now you understand why there is such a thing as Curie temperature; if heated enough the unpaired electrons will be removed from the iron atom.
And if electrons carry magnetic charge, you understand as when making permanent magnets while cooling them slowly down inside a strong applied outside magnetic field ensures the electrons will land there where it like to be.
And this, my dear reader, is an explanation of how permanent magnets work without the need for electrons that are glued into place. Electrons like to move around in their orbitals so once more if they were magnetic dipoles they could not hold on to a permanent state of magnetism…
__________
And so on and so on, my easy to understand insight the magnetism of the electron is much better compared to one century of physics professors.
Let’s leave it with that, thanks for your attention.