Molecular oxygen has the interesting property that it has a so called non-binding electron pair and it is know that in such a non-binding pair the electrons have the same spin. The way I view it for a couple of years is that the electrons are magnetic monopoles and that explains their behavior, so they don’t have a spin orientation so to say.
There is a relative simple experiment that I can’t do but if you have access to liquid oxygen and have some heavy electric magnets, you can do a simple experiment to see if the individual oxygen molecules behave like magnetic monopoles yes or no.
On Youtube there are plenty of videos showing the magnetic properties of oxygen by pouring the liquid stuff over a strong magnet or better: Pour it in between the two poles of two magnets.
After some time the oxygen will have separated over the two magnetic poles, if you can flip the magnetic polarity with the electric magnet, all oxygen should go loose and try to get to the other pole.
Once you have a setup like this or may be you have only strong permanent magnets, once the oxygen is separated you can try to put some of the liquid into a plastic bag. Let the oxygen become gas and see if the bag as a whole has magnetic monopole properties. That would be funny.
The only experiments I have done myself are those old ones with two televisions and try to that separation in the electron stream. It has to be remarked however that I once almost bought an old oscilloscope because you can let the electron beam go around so it becomes a circle on the glass tube. So that kind of experiment is still waiting…
Back to the oxygen molecule with it’s non-binding electron pair: Once I heard that I thought that likely oxygen must be in a lower energy state this way. May be that having an electron pair that pushes things apart give rise to a lower energy state. As far as I remember I never tried to look up the somehow more fine details.
So the video from the Action Lab came around for free and has all the information I needed. It has even information I was not waiting for like in the next screen shot:

In the next picture I grouped it a bit from four screen shots. And may I thank the folks from the chemical sciences for measuring all that energy stuff?
If the shapes below are correct, you see the molecule with the bonding pair becomes a linear shaped molecule that is clearly very different from triplet oxygen.
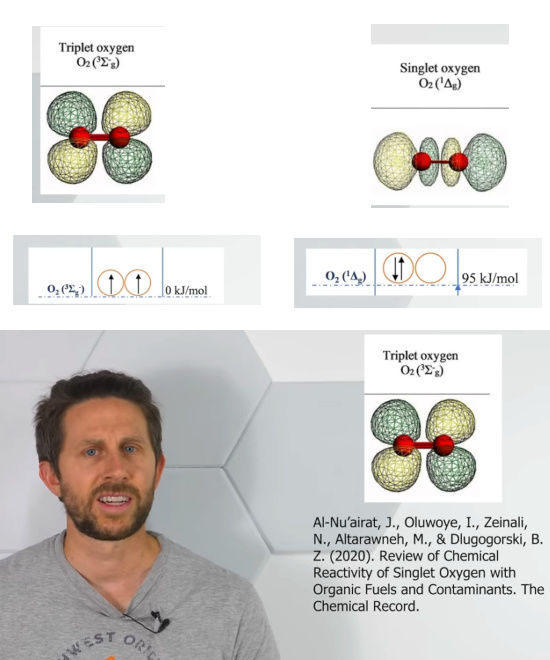
The video has the title Singlet Oxygen Is Scary! And yes there is something to say for a classification like that. Here is the video:
The reason as why I like this kind of stuff is that molecular orbitals make chopped meat of all that stuff that looks so sacred. Stuff like the Pauli exclusion principle, or the Aufbau principle and or the Hund rule for placing an extra electron. Stuff like that always assumes that all electrons are the same and it is just a matter of some magnetic vector pointing in this or that direction that solves the thing.
But I think electrons have a monopole magnetic charge that is permanent. So there are two kinds of electrons and that has all kinds of far reaching consequences. Well that is all for some other day.
In the meantime I am going to pop up a second beer, upload this post and after that I will ask the electrons in the beer the following question: Why do electrons never drink beer?
May be it is time to split. Goodbye.