Another short post, this time again on the totally crazy so called bonding and non-bonding pairs in theoretical chemistry. It is one of the many energy problems that come along if you want electrons to be tiny bipolar magnets. And if you view the electrons as magnetic monopoles, in that case all of a sudden you don’t have these kind of weird energy problems.
Lets dive into it, this post is 3 images long and here we go:


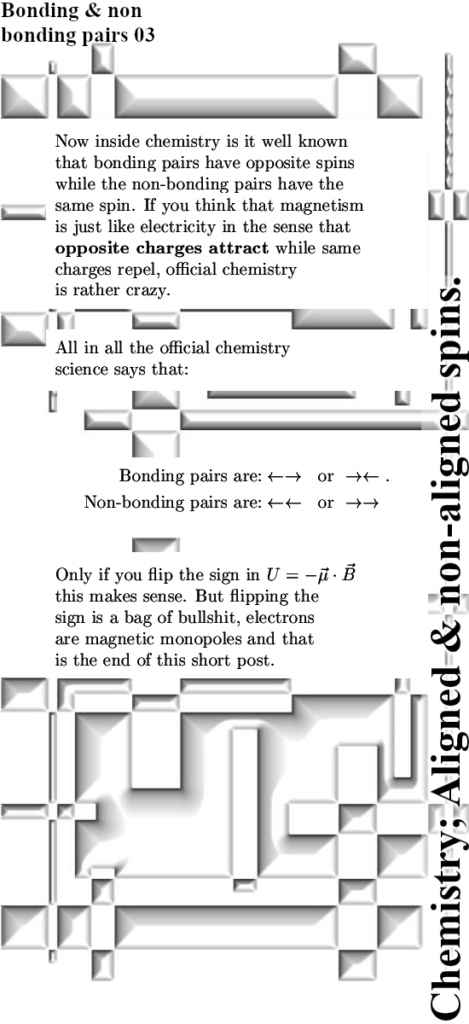
That’s more or less all I had to say today, if you view the magnetic properties of electrons as monopole magnets just like their electric properties the standard electron pair becomes the lowest (potential) energy state. Before I close this post let me quote from a wiki an interesting detail about the non-bonding electron pairs: they have a tendency to be outside the so called ‘bonding region’ between atoms in molecules. Once more this only makes sense when electron carry a monopole magnetic charge.
In theoretical chemistry, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes in the bonding region between the nuclei. The density of the electrons in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms. This is in contrast to a bonding molecular orbital, which has a lower energy than that of the separate atoms, and is responsible for chemical bonds.
Here is the link to the wiki I quoted from:
https://en.wikipedia.org/wiki/Antibonding_molecular_orbital
That’s it, see you in some other future post or enjoy some old posts on say the 4D complex numbers because they are beautiful and that is something we cannot say about the behaviour of the average physics professor with their weird fixation on electrons as tiny magnets…