Quantum computing looks like a good idea when it comes to simulation of quantum stuff like chemical reactions. But if your basic assumption of electrons being ‘tiny magnets’ it will all run from the rails if in the future it is found out that electrons are magnetic monopoles just like they are electric monopoles. Lately I have been joking that the only place in the universe where electron spin gets flipped is inside the heads of our professional physics professors.
I think there are two kinds of electrons, one with a magnetic north charge and the other with a magnetic south charge. For the time being I think this magnetic charge is permanent so there is no way or mechanism that turns a south charge electron in a north charged and vice versa.
Doing chemistry on a computer on the level of individual electrons and nuclei consumes an awful lot of computer resources. To focus the mind say you have a molecule with 100 to 150 electrons and some nuclei. The Coulomb forces alone are hard to simulate. But the way the magnetic forces must be done with the ‘tiny magnet’ model for electron spin is even much more horrible; in principle you have to calculate 100 to 150 vectors representing the bipolar magnetism of the electron. So this is all horribly complicated. Yet if electrons are magnetic monopoles, calculating a simulation for the magnetic forces should be of the same order of computer recourses as the Coulomb forces. That still is not very appealing but it is less worse as the Google engineers do it for the most simple atom there is in our universe: The hydrogen molecule made of two protons and one electron pair (the pair has opposite magnetic charges my dear reader, that is more logical as ‘opposite spins’ or for that matter ‘quantum numbers’).
The horror is that physics but also chemistry professors seem to think that in the electron pair every particle is in a super position of spin up and spin down. That is where that stuff like “If I separate the two quantum particles and I take one to the Andromeda galaxy and measure it’s spin in the vertical direction, instantantly the spin of the particle left behind will be the opposite“. And why don’t they never say that for a hydrogen atom? After all these are two quantum particles to but are these two particles that are both in a super position of their positive and negative electrical charges? Is the mass of both particles not defined but is either the proton mass or the electron mass? Most people will say that is mumbo jumbo.
The next picture is from a video I will show you below, likely all these Google people think that electrons are tiny magnets. So that is an amzing amount of salary costs wasted year in year out.

The Google view on electron magnetism is very different from mine where I like to keep it simple by stating electrons are magnetic monopoles. But they can make it much more complicated without any reason at all, in the next picture you see one of those singlet states and the chemistry folks have found out that an electron pair is ‘non binding’ if the two electrons have the same spin. If they have the same spin they don’t bond? And non of the chemistry weirdo’s remark that in a permanent magnet the spins must point in the same direction to make some magnetic bonding… Why does nobody see this is all not very logical and that this ‘tiny magnet’ stuff is just not true because it leads to all kinds of contradictions? Why are they so fucking stupid?
Let’s proceed with the second screen shot ensemble:
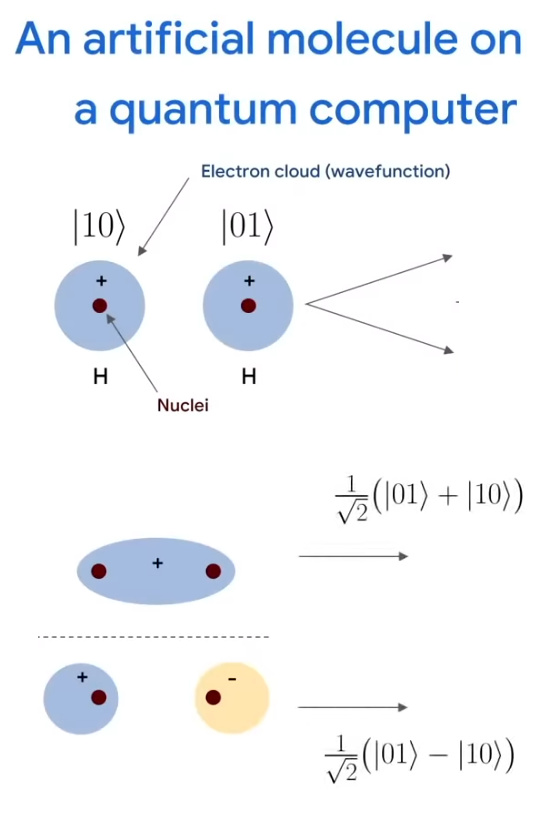
Lets try to hang in the Google video.
So far for this Google stuff. Luckily IBM is also very good at donating a coin of quantum wisdom. Lately we had a Noble prize in physics for faster than light quantum teleportation of quantum properties like photon properties… I am not saying all this Bell inequality stuff is impossible, all I am saying is an electron cannot be in a superposition of spin up and spin down because electrons carry a permanent magnetic charge.
The IBM video was so bad that it became funny and some kind of parody.
Here is another superposition of screen shots.
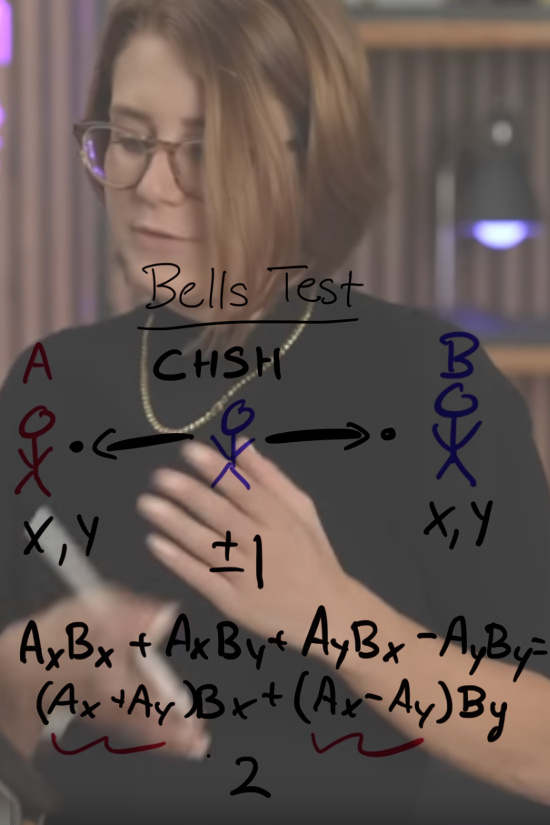
The IBM video:
End of this post.