I was watching a video about chiral chemistry and there it was explained that during the electrolysis of water if you coat the electrodes with some chiral coating, the efficiency is much higher. Now I was surprised but the explanation seems to be that oxygen has one so called non-binding electron pair. So that electron pair has two electrons with the same spin.
Lately I found out that chiral molecules are able of electron spin selection, I have to admit I don’t understand just one tiny detail of how that works but on the other hand physics professors don’t understand electron spin. So compared to that I am doing fine.
For readers who are relatively new: During the last 7 or 8 years I have been trying to kill the insight that electrons are magnetic monopoles. And all that difficult doing of the professional physics and chemistry professors is just plain crap. If electrons are magnetic monopoles, in that case it is of little use to describe the energy in terms of inner products of spin vectors. So a lot of physics models on magnetism are completely crap.
Anyway, oxygen is at first look totally not magnetic. But it seems that chemistry is not a total chaotic science at all because according to their moleluclar orbital theory it is explained that an O2 oxygen molecule has two unpaired electrons. See the next picture:
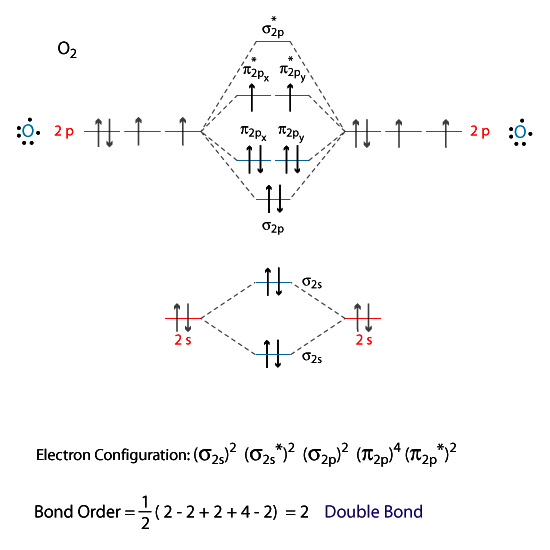
You can think of many experimental designs in order to prove that electrons are magnetic monopoles. If my insights are correct, each and every O2 molecule must act as a magnetic monopole. That means if you apply the correct magnetic field, you can repel oxygen molecules and attract the same molecules if you flip the applied magnetic field.
If you remark that if oxygen molecules act as magnetic monopole molecules, the scientists would have found out that decades ago…
But my dear reader that is not how the human mind works. If the belief is that magnetic monopoles do not exist, that causes the mind to be blind as why electrons or oxygen molecules behave as they do.
For example the electron pair is neutral when it comes to magnetic fields, how do the professional professors explain that? Well they say that the opposite magnetic fields cancel each other out. So why don’t they see they are telling crap? If the electron is a tiny magnet with a north and a south pole, isn’t that magnetically neutral to begin with? So why is the unpaired electron not magnetically neutral? At that point they begin telling stuff like ‘anti alignment’, the Pauli exclusion principle and most of all ‘quantum numbers’. It all doesn’t add up, it is crap.
That is what I had to say on this tiny detail: Likely each and every oxygen molecule with such a non binding electron pair in it will act as a magnetic monopole…