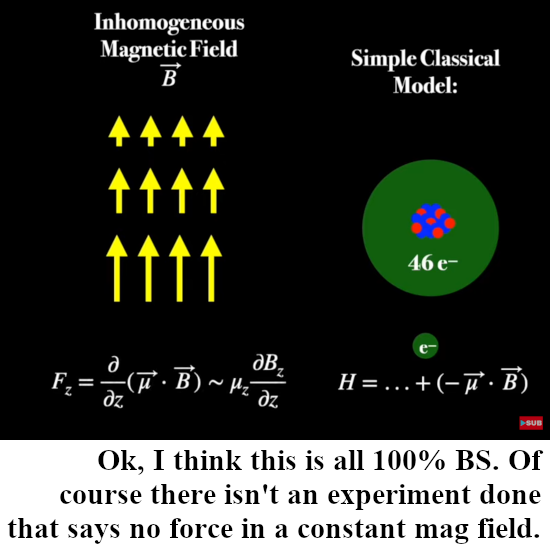
One or two days back this video from Dr. Jorge S. Diaz came out and all in all in it’s kind it is very good. Even for me there is a lot of new stuff in although nothing of the real important things like why an inhomogeneous magnetic field was used: Otto Stern thought that the silver atoms themselves would act like tiny magnets because of the electrons going round the nucleus. I want to remark that using an inhomogeneous magnetic field when it comes to atom sized magnets makes sense, where I draw the line is the blind application to a point like particle like the electron.
So all the big hammers were already known to me yet there is a lot of cute stuff in it I had never seen. Things like the first introduction of those quantum numbers from the principle n to the magnetic number m.
In the video Jorge Diaz shows once more what the physics people use as the potential energy when a dipole magnet is placed in a magnetic field, you can see that in the picture below.
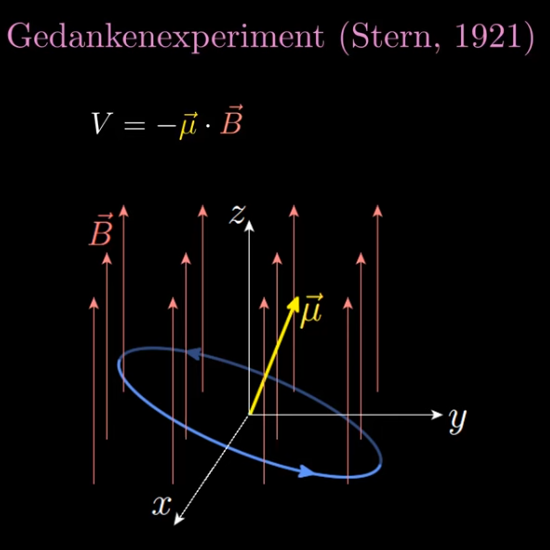
It is well known that nature loves to minimize the potential energy and here this is the case if both vectors mu and B point in the same direction. In that case the inproduct is a positive number and the minus sign guarantees the minimum of potential energy.
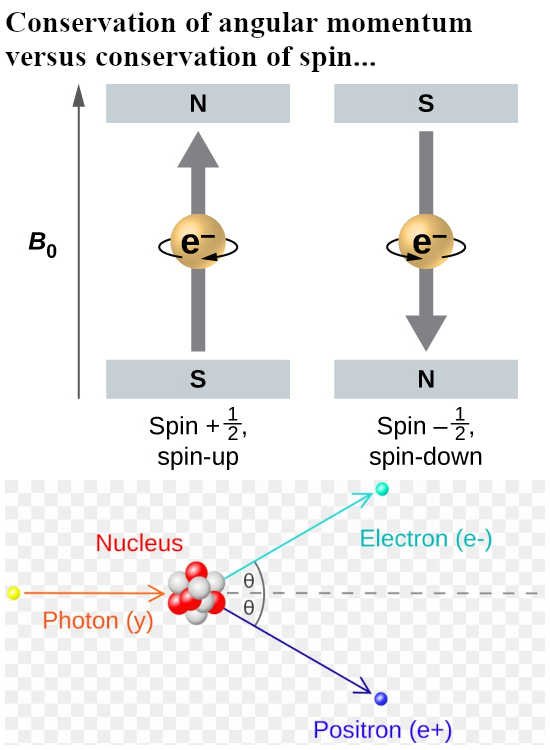
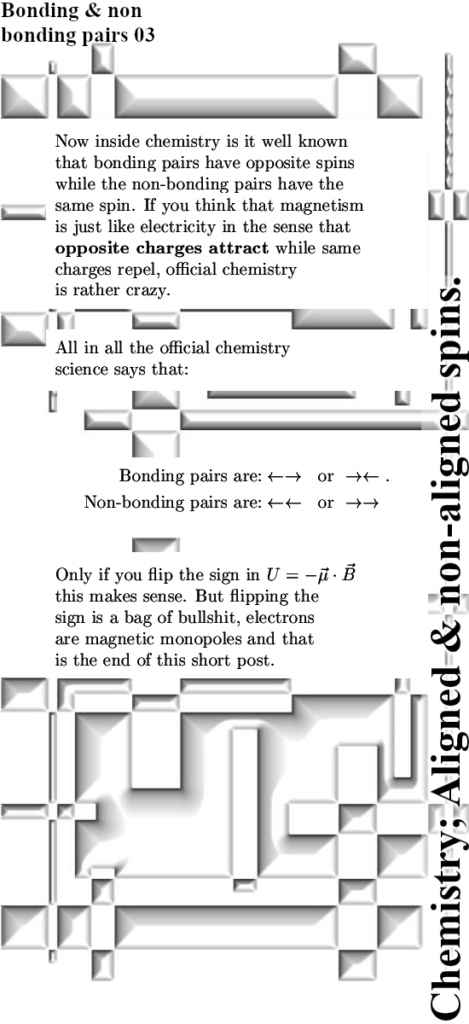
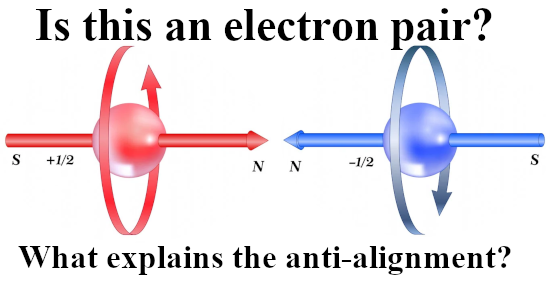
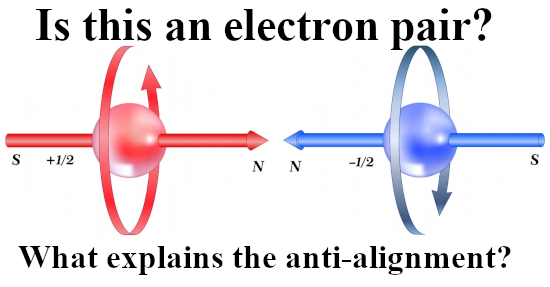
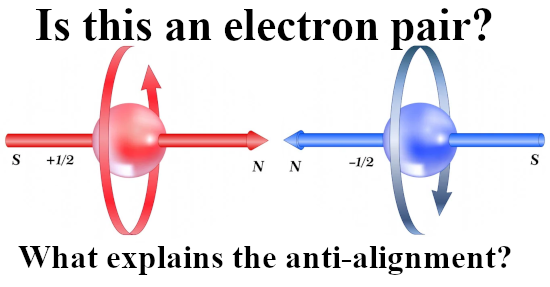
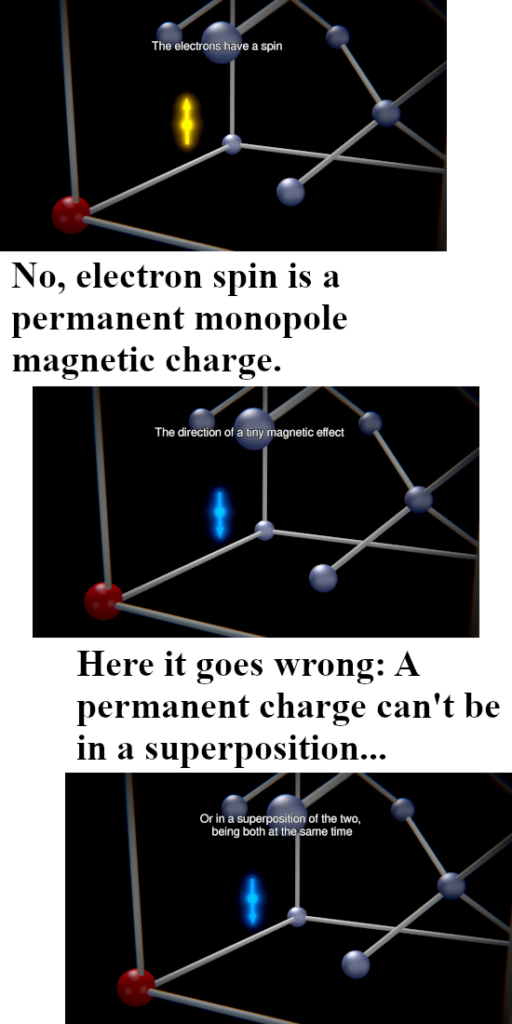
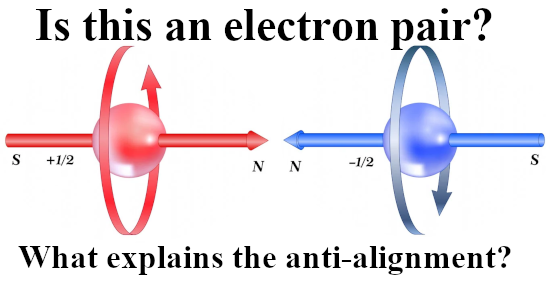
Last year I made a picture for repeated use during this year 2024 and in it you see the official version of an electron pair. The Pauli exclusion principle says that the magnetic numbers must differ and as such they must have opposite or anti-parallel spins. The whole problem is of course that if you calculate the potential energy where you view one spin in the magnetic field of the other and use the above expression, you get a positive potential energy. That’s weird since in the science of chemistry it is well known that the electron pair plays an important role in forming atomic and molecular bonds. Here is the sketch of the electron pair once more:
I made a similar picture for a lone electron that anti-aligns itself with an applied magnetic field:
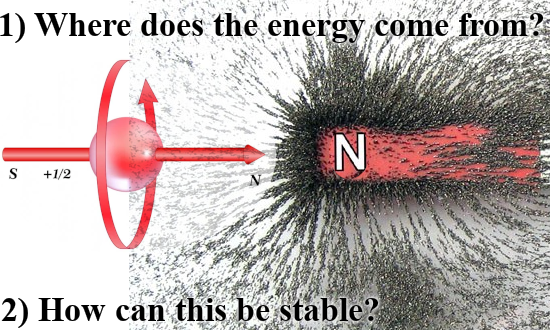
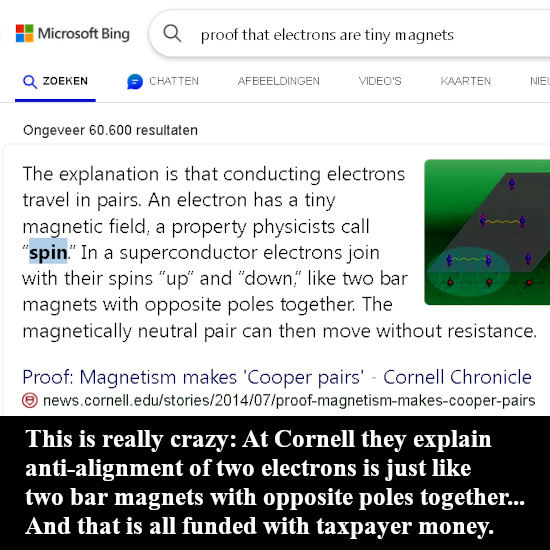
As you can see for yourself in the video below, people like Jorge Diaz never even mention that there are severe energy problems. I name that avoiding Crazyland, they only explain the things that sound logical and as soon as it becomes absurd like here with the electron pair, they just don’t talk about that.
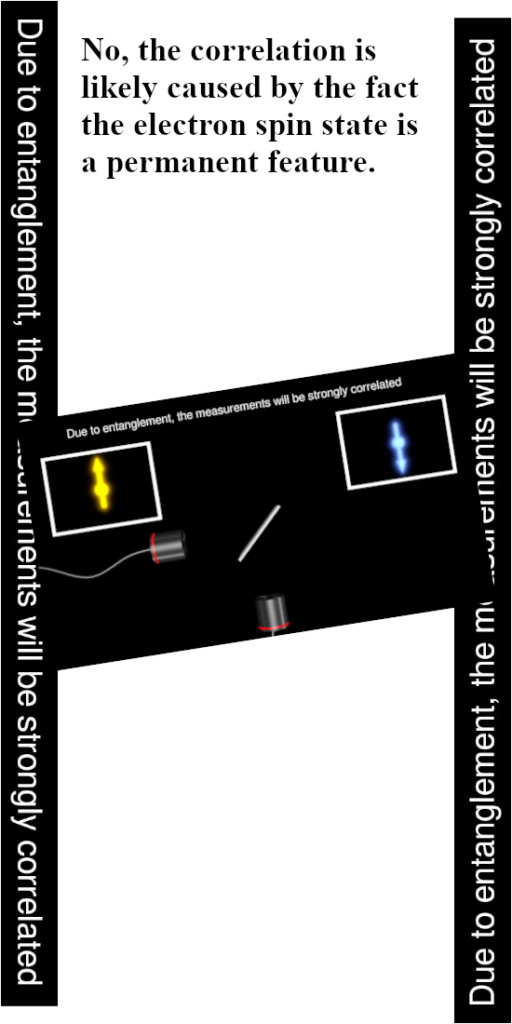
The weird potential energy problems arise only if you think the electron is a tiny magnet. Since the year 2015 every year I became a bit more convinced that electrons are magnetic monopoles just like they are electric monopoles. All energy problems fade away fast if you do that but hey try to explain that to people like Jorge Diaz! Or for that matter all those other professional physics people out there, those weirdo’s also think that magnetic monopoles do not exist so the taks of explaining things to those people is an almost impossible task.
I also combined a few screen shots from the video with people that played some role or contributed to the Stern-Gerlach experiment. For myself I more or less like it that even a guy like Albert Einstein never realized the monopole nature of electron magnetism. But I am also well aware that this can work against me; the physics professionals will likely think that if Albert didn’t see it, it can’t be true and as such for themselves they have once more confirmed that magnetic monopoles don’t exist…

And finally the video, again in it’s kind it is a very good video:
A lot more could be said or written but lets not do that and may I thank you for your attention.